Precision therapies for respiratory diseases
Dedicated to the development of novel respiratory therapies to address urgent unmet needs

Our Mission
Atelerix Life Sciences is focused on addressing unmet medical needs in the prevention and treatment of respiratory diseases and threats by leading novel scientific research that will change clinical practice and save lives.
We are advancing multiple pre-clinical and clinical-stage therapies with life-saving potential in three critical areas of need:
Drug-induced respiratory depression (DIRD) in surgical, ICU, and emergency medicine settings
Respiratory disease exacerbations such as asthma and COPD
Bio-defenses against aerosolized opioids, viruses, and other respiratory threats
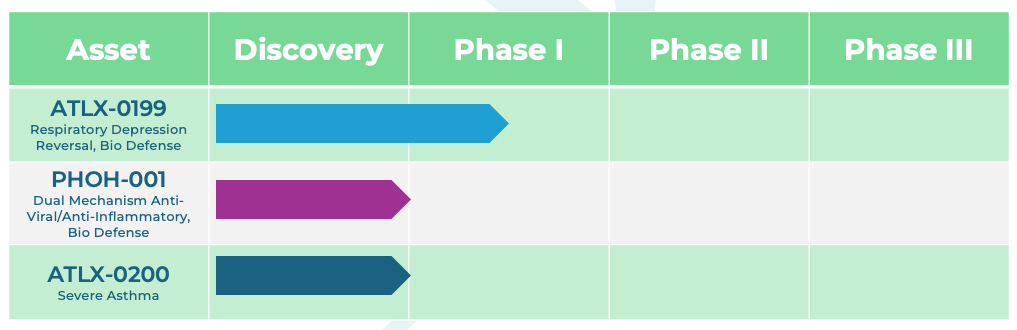
Our Pipeline
Building a portfolio of Phase I–ready assets with potential in respiratory diseases and bio-defense
ATLX-0199: A Novel Respiratory Stimulant for DIRD and Bio-Defense
ATLX-0199 is a novel respiratory stimulant being developed to address the morbidity and mortality arising from drug-induced respiratory depression (DIRD) and related conditions
- DIRD is considered a serious medical condition, leading to significant morbidity or mortality if not reversed
- ATLX-0199 is unique – there is no reversal agent commercially available that does not work on the opioid receptor
- ATLX-0199 has been identified as the lead molecule from within a novel class of proprietary compounds that stimulate respiratory drive while preserving the analgesic effects of opioids
- In addition to its hospital use, ATLX-0199 could drive a paradigm shift in the world’s ability to counter a respiratory depressant terrorist attack
Why DIRD Matters
Drug-induced respiratory depression (DIRD) is a common, costly, and potentially life-threatening condition for which there is no current approved treatment that preserves pain relief.
What is drug-induced respiratory depression?
A reduction in minute ventilation
A common and potentially deadly side effect of opioids and sedatives
Occurs in many settings, including ICU, hospital operating rooms, and the community
A Unique Mechanism of Action
ATLX-0199 enhances the drive to breathe while preserving sedation and pain relief.
- Targets pathways in the carotid body and brainstem via a unique MOA
- Not an opioid receptor antagonist; does not work at the mu opioid receptor
- Efficacy can be achieved with a single IV dose
- Preserves the analgesic effects of opioids
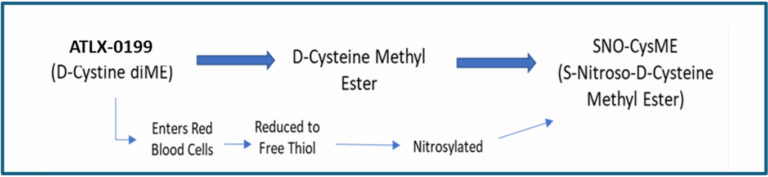
D-cystine dimethyl ester (ATLX-0199)

ATLX-0199 (chemical name D-Cystine di-methyl ester)
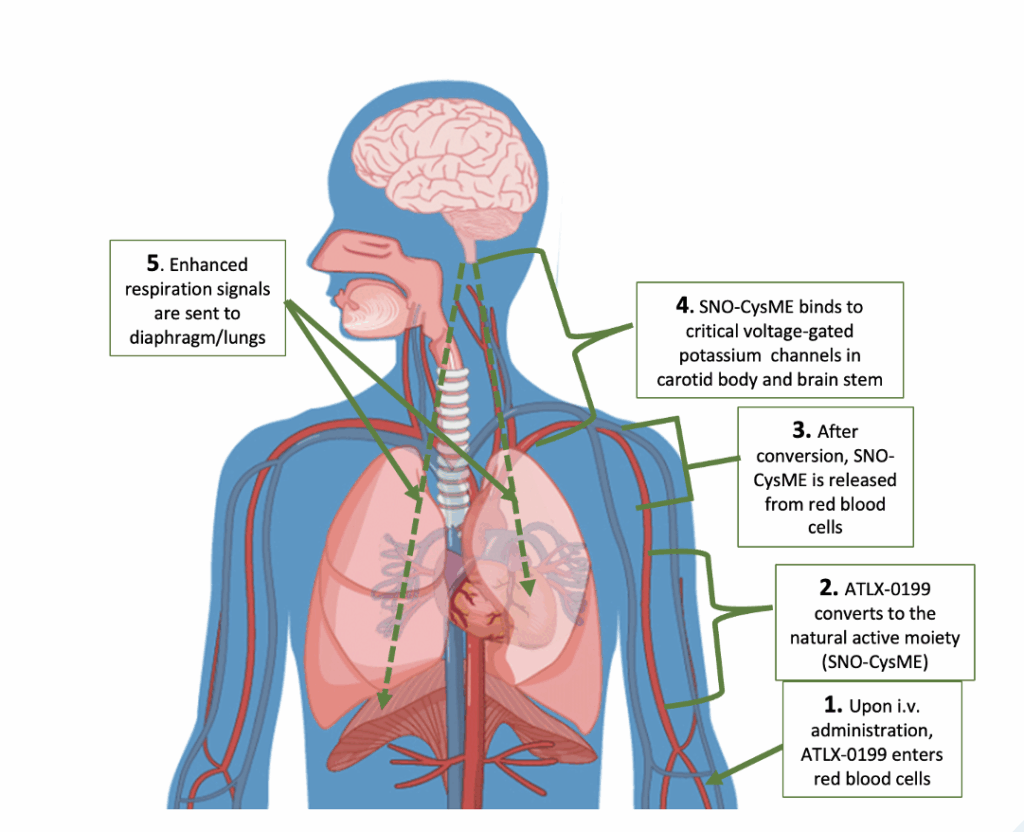
From Prodrug to Natural Moiety
ATLX-0199 (D-Cystine diME) is a prodrug that, when taken up by red blood cells following i.v. injection, converts to the potent natural active respiratory stimulant SNO-CysME. When released from the red cells, SNO-CysME binds to critical voltage-gated potassium channels in the carotid body and brain stem, sending neural signals that enhance respiration and reverse OIRD.
A Broader Mission: Addressing Respiratory Threats
Bio-Defense Applications
ATLX‑0199 is designed to address more than just surgical risks — it’s being developed as part of a new generation of countermeasures against:
- Aerosolized opioids (e.g., fentanyl)
- Viral respiratory threats
- Chemical or bioterrorism-induced respiratory depression
With potential for both hospital-based and field deployment, these therapies represent a strategic approach to public health and emergency preparedness.
ATLX-0199 is potentially an important defense against the threat of aerosolized high potency synthetic opioids, and early evidence suggests that it will also work against multiple respiratory depressant drugs.

Unmet Need. Urgent Opportunity.
Millions are at risk — and today’s options fall short.
Each year, millions of patients in surgical, ICU, and emergency settings are exposed to respiratory depression caused by opioids, sedatives, or combinations of both. Outside the hospital, exposure to aerosolized fentanyl and other agents is a growing public health and national security concern.
- 1 in 15 surgical patients experience opioid-induced respiratory depression (OIRD)
- 1 in 7 ICU patients experience drug-induced sedation-related breathing compromise
- No approved agent currently reverses OIRD without blocking pain relief
ATLX-0199 is being developed to change that — preserving respiratory drive while maintaining analgesia.

Addressing ICU and surgical risk across millions of patients — and billions in systemic costs.
Intellectual Property
ATLX-0199 has robust IP out to 2040 – with early opportunities to expand and strengthen
Currently four patents filed which cover Composition of matter, Compositions and methods for stimulating ventilatory and or respiratory drive
Patents files in Jan 2018, Sep 2018, Jun 2020, Aug 2024 so provide cover to beyond 2040
We are seeking to file further patents covering CoM and MoU for the lead compound and a follow-on compound
Additional patents being pursued on formulation design and separate routes of administration in Hospital and Community settings
Experienced leadership team
Executive Committee
Board of Directors
Scientific Advisors

Glenn Tyson
Chief Executive Officer

Susan Learned
Chief Medical Officer

Herb Gaston
Chief Operating Officer

Ian Crouch
Talent and Investor Relations

Benjamin Gaston
Chief Scientific Officer

Peter Hutt
Board Member

William Akridge
Board Member

Benjamin Gaston
Board Member

Benjamin Gaston
Founder

Steven Lewis
Founder

Harry N Cook
VP of Drug Development

James Bates
Founder
Get In Touch
- Info@atelerixlifesciences.com
-
Atelerix Life Sciences Inc.
1800 N Capitol Ave Noyes Pavilion, 5th Floor, Suite E504-ATLX Indianapolis, IN 46202
