About Us
Introduction
Unmet Need: Every year, millions of US surgical patients experience opioid-induced respiratory depression (OIRD) severe enough to require prolonged mechanical ventilation and longer hospital stays.
- Problem: Current drug for OIRD, Naloxone, restores breathing but is problematic, and therefore used infrequently, because it reverses pain relief
- Solution: New drug, ATLX-0199 (sometimes referred to as Sudaxine), reverses OIRD while preserving pain relief
- Market: Over $13 billion annually
- Technology: Proprietary small molecule platform; strong animal data; NIH-backed
- Team: Experienced, industry-known, motivated, committed
- Stage: Late pre-clinical

Opioid Side Effects
While useful in controlling pain, opioids have significant limitations:
Opioid-induced side effects have created unmet medical needs of crisis proportion, affecting millions of people annually.
- Life-threatening respiratory and cardiovascular reactions
- Withdrawal/tolerance/addiction
- GI (motility) disruption
- Potential for mass casualties
A Better Solution
ATLX-0199, an active thiol-based compound from our platform of novel drugs, reverses Opioid-Induced Respiratory Depression (OIRD) while preserving pain relief:
ATLX-0199 targets novel molecular pathways, modulating signals downstream from activated opioid receptors. Concept validated by extensive NIH-supported research/data. Atelerix is developing ATLX-0199 and other compounds in its pipeline to treat the range of opioid-induced side effects; first focus is OIRD in the hospital surgical/ICU setting.
Opioid-Induced Respiratory Depression (OIRD)
- OIRD is a life-threatening side effect of opioids characterized by respiratory depression or cardiac collapse
- It affects millions of hospital patients every year
- Opioid-related advers drug events result in average increased hospitalization costs of $8,225 per patient
- Our solution addresses a multi-billion dollar market
Weaponization
The opioid crisis has a new and even uglier dimension – weaponization – and the focused funding interest of the NIH and the United States military.
“These public health threat agents are highly toxic chemicals that could cause mass casualties after either deliberate terrorism-related release or inadvertently.”
– National Institutes of Health
Russian special forces soldiers are seen carrying out hostages killed by a highly potent weaponized opioid in a Moscow theatre after a three-day stand off with Chechen terrorists.
(Credit: Anton Denisov/AFP/Getty Images)
Addiction / Tolerance / Overdose / Withdrawal
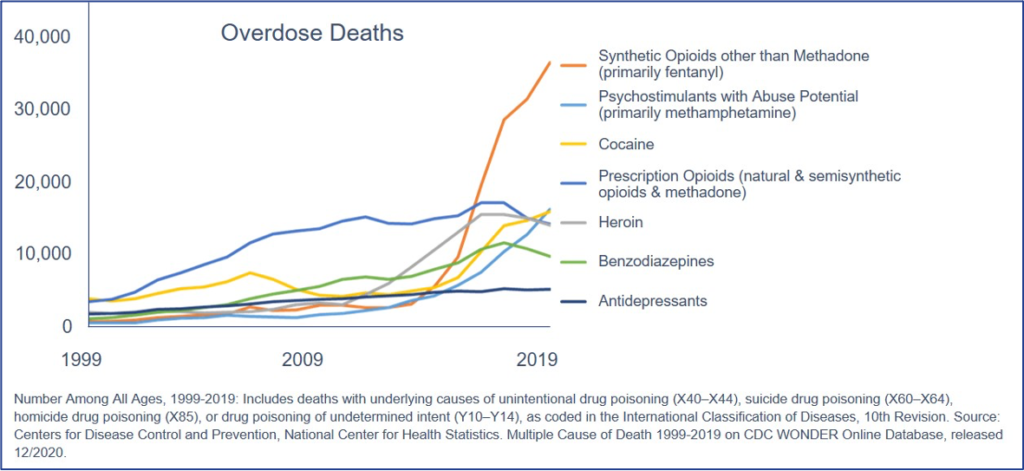
Overdose deaths from synthetic opioids (primarily fentanyl) have become a massive crisis, approaching 40,000 in 2019, and growing since COVID-19. Atelerix’s novel approach to opioid-induced unmet medical needs could diminish the number of opioid-related deaths.
Current Standard has Significant Disadvantages
Naloxone restores breathing but also:
- Immediately reverses opioid analgesia, leaving surgical patients in pain
- Short-acting, requiring frequent re-administration
- Less effective against highly-potent, synthetic opioids
- Can cause cardio-pulmonary arrest
ATLX-0199 Target Market
Opportunity for OIRD in surgical/ICU setting is $13 billion annually
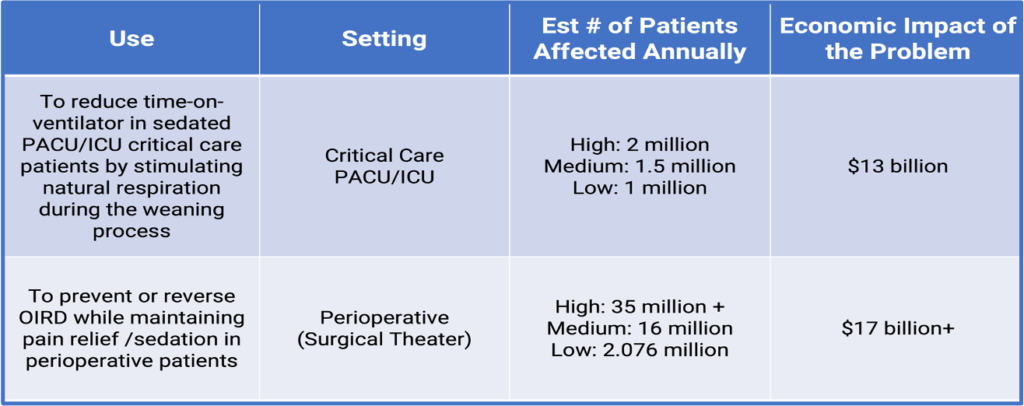
USE 1: 20-40% of ICU patients require mechanical ventilation. To estimate the number of patients, we use 40% (2 million) for the “high” estimate, 30% (1.5 million) for “medium,” and 20% (1 million) for “low.” 40% of time in ICU is weaning from ventilator. Patients requiring mechanical ventilation spend a mean duration of 5.6 days in the ICU. We expect ATLX-0199 to reduce time in ICU by 2.24 days (40% of 5.6 day mean stay). Mechanical ventilation beyond day 3 in the ICU costs an average of $3,968 per day. Reducing ICU stays by 2.24 days could yield a reduction in systemic costs by over $13 billion ($3,968 x 2.24 days x 1.5 million patients.)
USE 2: Approximately 88% (35 million+) of surgical patients receive opioids to control their pain. 46% (16 million+) of patients receiving opioids experience some Opioid-Induced Respiratory Depression. 5.19% (2.076 million) of patients experience OIRD of a severity sufficient to be designated a respiratory-related Opioid-Related Adverse Drug Event. ORADEs result in average increased hospitalization costs of $8,225 per patient. $8,225 multiplied by 2.076 million patients expected to experience an ORADE yields a total systemic burden of over $17 billion a year.
Grant Funding to Date
Over $16 million in prior non-dilutive funding to Atelerix Scientific Co-founders. $1.84 million SBIR grant awarded to Atelerix in 2022.

Company has exclusive option on resulting intellectual property
